Four Fold Serial Dilution
Two Methods: A dilution in chemistry is a process that reduces the concentration of a substance in a solution. A serial dilution is the repeated dilution of a solution to amplify the dilution factor quickly. It’s commonly performed in experiments requiring highly dilute solutions with great accuracy, such as those involving concentration curves on a or involving experiments to determine density of bacteria. Serial dilutions are used extensively in experimental sciences like biochemistry, microbiology, pharmacology and physics. Backuptrans Iphone Whatsapp Transfer Keygen Free. Nascar Heat Skin Template Photoshop. Prepare several test tubes with 9 mL of dilution liquid. These tubes will serve as your dilution blanks.
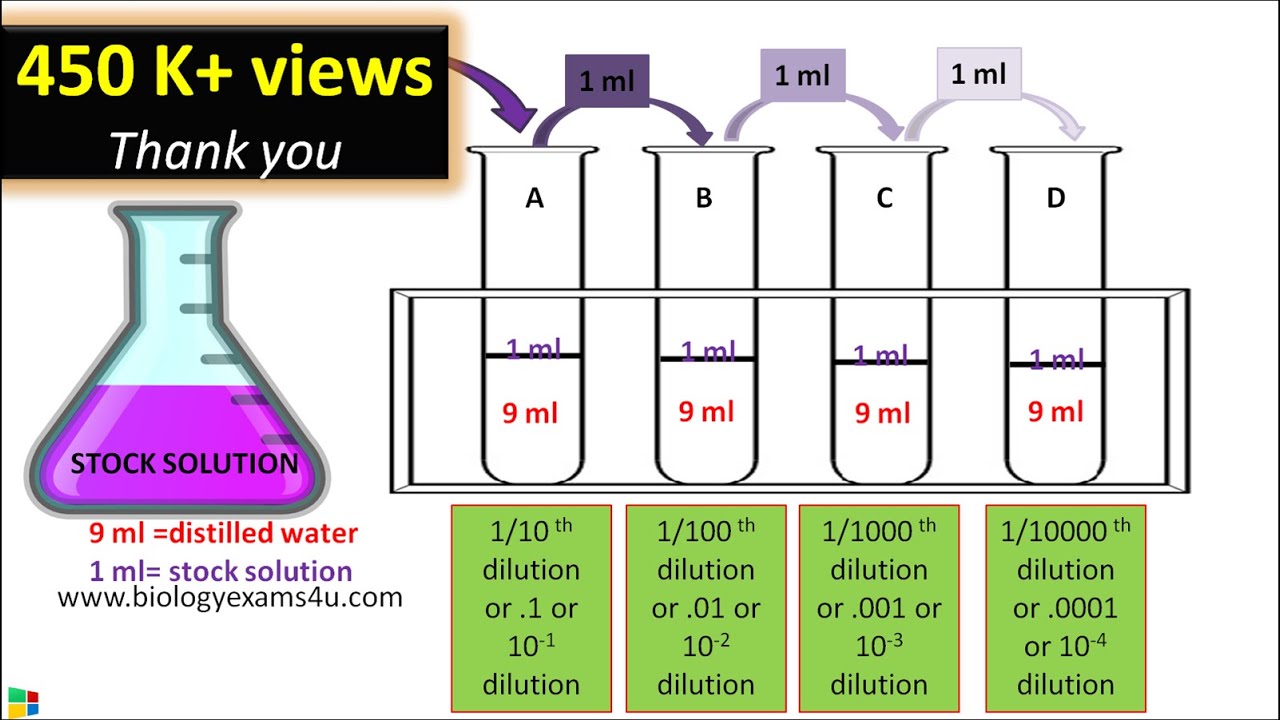
You will be adding your undiluted sample to the first tube and then serially diluting into the following tubes. • It’s helpful to label all of your tubes before you begin so you don’t get confused once you begin with the dilutions. • Each tube will be a 10-fold dilution starting from the undiluted tube. The first tube will be a 1:10 dilution, the second a 1:100, the third a 1:1000, etc. Determine the number of dilutions you need to do beforehand so you don’t waste tubes or diluting liquid. Calculate the final dilution in a serial dilution. The total dilution ratio can be determined by multiplying the dilution factor of each step leading up to the final step.
Ten-fold serial dilutions. A ten-fold dilution reduces the concentration of a solution or a suspension of virus by a factor of ten that is to one-tenth. 2-fold dilution is a bit confusing, a better way of describing dilution is ratio. For 10 fold serial dilution, you do the above 10 times. Serial Dilutions A serial dilution is any dilution where the concentration decreases by the same quantity in each successive step. Serial dilutions are mutiplicative.
This can be mathematically illustrated with the equation D t = D 1 x D 2 x D 3 x x D n where D t is the total dilution factor and D n is the dilution ratio. • For example, let’s say you did a 1:10 dilution of your liquid 4 times. Plug your dilution factor into the equation: D t = 10 x 10 x 10 x 10 = 10,000 • The final dilution factor of the fourth tube in your serial dilution is 1:10,000. The concentration of your substance is now 10,000 times less than the original undiluted solution. Determine the concentration of the solution following dilution. To determine the final concentration of your solution following serial dilution you will need to know your starting concentration.
The equation is C final = C initial/D where C final is the ending concentration of the diluted solution, C initial is the starting concentration of the original solution and D is the dilution ratio previously determined. • For example: If you started with a solution of cells with a concentration of 1,000,000 cells per mL and your dilution ratio is 1,000, what is the final concentration of your diluted sample? • Using the equation: • C final = C initial/D • C final = 1,000,000/1,000 • C final = 1,000 cells per mL.
• Tell us some more • Upload in Progress • Upload failed. Please upload a file larger than 100x100 pixels • We are experiencing some problems, please try again. • You can only upload files of type PNG, JPG, or JPEG. • You can only upload files of type 3GP, 3GPP, MP4, MOV, AVI, MPG, MPEG, or RM. • You can only upload photos smaller than 5 MB.
• You can only upload videos smaller than 600MB. • You can only upload a photo (png, jpg, jpeg) or a video (3gp, 3gpp, mp4, mov, avi, mpg, mpeg, rm). • You can only upload a photo or a video. • Video should be smaller than 600mb/5 minutes • Photo should be smaller than 5mb •.